By Tom Travis
Shatarian Aliger, 35, an entrepreneur/fashion designer, said she overcame her COVID vaccination hesitancy out of concern for her family. Thursday, she got her second Moderna shot in the Flint Township office of Dr. Bobby Mukkamala. She had received her first dose July 13.
“I was hesitant. I am the last one in my family to receive the vaccine,” she said. Her reluctance, she said, was due to hearing things on the news and the problems that occurred with the Johnson & Johnson vaccine.
“I did want to wait and see what was going to happen,” Aliger said. But with the recent rise of the Delta variant, Aliger said, “I thought it was important to keep my family safe, especially my children.”
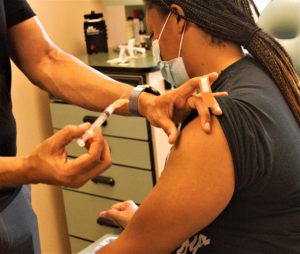
Shatarian Aliger, 35, receiving her second dose of the Moderna vaccine by Dr. Mukkamala. (Photo by Tom Travis)
Aliger said her niece, a hospital employee, once tested positive for the COVID-19 virus. Aliger added a member of her extended family died from the virus, but “no one close to me.”
Asked what helped her overcome her hesitancy, Aliger responded, “Mainly the desire to protect my children and family. I have an 86-year-old grandmother and an 87-year-old grandfather and a great-aunt who is 83. I wanted to take the precautions so that I could be with my family and protect people that I’m with and pass by every day.”
What she would say to those who are hesitant about getting the vaccine? “Definitely do your research,” Aliger said. “It is up to you. I recommend getting [the vaccine] for your own health and for the health of your loved ones.”
,James Henthorn, 19, a computer science major at Michigan Tech, received the the Johnson & Johnson vaccine in early April and Thursday received a Moderna booster vaccine.
Mukkamala said the Moderna booster shots are being administered in California to those who received the Johnson & Johnson vaccine. The other vaccines cannot be given interchangeably. However, if you have the Johnson & Johnson vaccine you can receive the a booster from one of the other vaccines.

James Henthorn, 19, receiving a booster vaccine of Moderna after receiving the Johnson & Johnson vaccine in April. Dr. Mukkamala is administering the vaccination in his Flint Township office. (Photo by Tom Travis)
Henthorn said he received the booster today because it was recommended to him and he wants to continue to protect himself and others when he begins school this fall.
Mukkamala spoke to EVM in his office in Flint Township about his efforts to help with vaccinations in Genesee County. In addition to giving shots in his office, Mukkamala said he keeps vaccines in his car and is always ready to vaccinate those who are willing. The day before he was in Buckham Alley, downtown Flint, behind The Loft Bar, and vaccinated about 10 young people.
Mukkamala recalled that when the vaccines first became available in early 2021, he volunteered at large vaccination sites around the county and would often have 1,000 people a day. “Those who wanted to get the vaccine have received it and we have to work harder at engaging those who are hesitant,” he said.
Concerning the hesitancy towards the vaccine,Dr. Mukkamala said sometimes it’s a genuine and legitimate fear because it’s new and it hasn’t been around for five years. He acknowledges that is a “plausible reason that someone might be hesitant.”
But in addition, there is what he calls “the illegitimate fear, like [the vaccine is] going to mutate your DNA or that the ingredients of the vaccine are going to be in your system six months from now causing such-and-such a side effect.”
“Apathy” towards vaccinations is another reason for hesitancy, he added, along with being misinformed about the facts of the science behind the vaccines.
Grim vaccination rate for the city of Flint – 35 per cent
Genesee County is about 52.5 percent fully vaccinated against COVID-19 — a total of 182,073 residents — as of Aug. 11, according to figures shared this week by the United Way of Genesee County (UWGC).
The rates within the City of Flint, however, are lower. Data from the MDHHS show that within the city limits vaccination rates fall below 35 percent, making it among the lowest vaccinated percentage in the state. Townships surrounding Flint vary, with vaccination rates between 35 and 50 percent.
That compares to 59 percent fully vaccinated — 5 million residents — for the state of Michigan overall, according to the Michigan Department of Health and Human Services (MDHHS).
Those numbers, along with other statistics and related vaccination information, are being provided to local organizations and the media through a new $134,000 grant to the UWGC from the federal government’s COVID relief funds, according to CEO Jamie Gaskin.
Genesee County is among 20 communities statewide identified with high hesitancy among its residents to receiving the COVID vaccine.
Oakland and Washtenaw counties have among the highest vaccination rates in the state at 69 percent. Leelanau and Emmet counties, in the north west corner of Michigan’s lower peninsula, have greater than 70 percent vaccination rates.
Nearby, Livingston is 61 per cent, Shiawassee is 53 per cent, Saginaw is 55 per cent, Tuscola and Lapeer are 48 per cent. The graphic below from MDHHS shows a close up, with a key, of the low vaccination rates in the county and within the City of Flint.
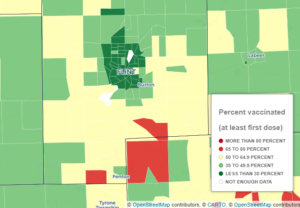
(Source: MDHHS)
FAQs on COVID answered here
Organizations receiving United Way funding from the COVID-relief grant were required to attend a Zoom training session in which the vaccine was explained and community leaders were given guidance on how to help those with vaccine hesitancy.
A “COVID-19 Communication Guide” was offered, created in partnership with the Center for Health Communications Research, Rogel Cancer Center of Michigan Medicine, Michigan Communities Conquering COVID-19 and Michigan Primary Care Association (MPCA).
“The training was brought to the UWGC grantees through the Michigan Association of United Ways and Genesee County United Way. The Greater Flint Health Coalition added local content from reputable sources including the Centers for Disease Contro(CDC) and John’s Hopkins University,” according to Janee Tyus of the Greater Flint Health Coalition.
The COVID-19 communication guide included information about the three types of COVID vaccines, how the vaccines work, how the vaccines were developed, and how effective are the vaccines. The content below in this article comes largely from that COVID-19 communication guide and the CDC.
Three vaccines are currently under Emergency Use Authorization (EUA) from the U.S. Food and Drug Administration (FDA): Pfizer, inc/BioNTech for ages 12 and older, Moderna for ages 18 and older, and Johnson & Johnson for ages 18 and older.
Among the materials offered were answers to commonly asked questions, including:
How do the vaccines work?
All vaccines teach the body to develop an immune response to fight against the COVID-19 virus. The Pfizer and Moderna vaccines use mRNA in their vaccines.
“mRNA vaccines are a new type of vaccine to protect against infectious diseases. To trigger an immune response, many vaccines put a weakened or inactivated germ into our bodies. Not mRNA vaccines. Instead, they teach our cells how to make a protein—or even just a piece of a protein—that triggers an immune response inside our bodies. That immune response, which produces antibodies, is what protects us from getting infected if the real virus enters our bodies,” according to the CDC.
The Johnson & Johnson is viral vector vaccine delivers these instruction inside the harmless shell of the common cold virus. The CDC explains how the Johnson & Johnson vaccine differs from the mRNA (Pfizer and Moderna) vaccines.
Viral vector vaccines use a modified version of a different virus (the vector) to deliver important instructions to our cells.
First, the vector (not the virus that causes COVID-19, but a different, harmless virus) will enter a cell in our body and then use the cell’s machinery to produce a harmless piece of the virus that causes COVID-19. This piece is known as a spike protein and it is only found on the surface of the virus that causes COVID-19.
Next, the cell displays the spike protein on its surface, and our immune system recognizes it doesn’t belong there. This triggers our immune system to begin producing antibodies and activating other immune cells to fight off what it thinks is an infection.
At the end of the process, our bodies have learned how to protect us against future infection with the virus that causes COVID-19. The benefit is that we get this protection from a vaccine, without ever having to risk the serious consequences of getting sick with COVID-19. Any temporary discomfort experienced after getting the vaccine is a natural part of the process and an indication that the vaccine is working.
How many shots will I need?
With the Pfizer vaccine two shots are needed with 21 days between each injection. The Moderna vaccine requires two shots with 28 days between each injection. The Johnson & Johnson is the “one and done” vaccine with only a single injection needed. People are considered fully vaccinated two weeks after their last dose. The vaccines provide protection for at least six months and some researchers believe the protection period is longer.
How effective are the different vaccines?
In clinical trials in the U.S., the Pfizer and Moderna vaccines were about 95% effective at preventing illness caused by the COVID-19 virus. The clinical trial of the Johnson & Johnson vaccine was done in several countries. It was 66% effective overall (72% effective in the U.S.). Researchers and the medical community almost unanimously agree that the vaccines work very well, nearly 100% at preventing death or the need for hospitalization from COVID-19.
What are COVID-19 variants?
Over the course of the pandemic, several variants of the COVID-19 virus have appeared and spread. Variants can spread more easily and can cause more serious infections and present different symptoms.
How do we know if the COVID-19 vaccines are safe?
The COVID-19 vaccines were tested in large clinical trials that included tens of thousands of people. The vaccines were developed quickly because lots of people volunteered for vaccine trials. No steps were skipped in the development of the vaccines.
Ongoing vaccine safety monitoring is being conducted by the National Vaccine Adverse Event Reporting System (VAERS) and the CDC V-Safe Vaccine Safety Tracker.
EVM Managing Editor Tom Travis can be reached at tomntravis@gmail.com.

You must be logged in to post a comment.